| time
requ.: |
20 minutes. |
|
objective: |
Demonstration
of oxygen as an air component. |
| material: |
- 1-2 syringes 50ml
- glass tube approx. 0,8*20cm
- 1-2 stoppers
- air balloon
- adhesive film
|
- test tube d=30mm
- beaker
- wooden splint
- burner
- lighter
|
| chemicals: |
|
|
|
procedure1: |
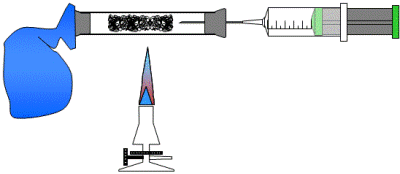
The glass
tube is fluffily filled with iron wool and closed on one side with the
stopper which is penetrated by a
syringe filled with 50ml of air, the other side with an empty
air balloon fixed with adhesive film (instead a second syringe may be
used). Heat the iron wool and move the air from syringe to balloon
and back. Repeat until the volume remains constant. |
|
observation 1: |
The volume of
the gas is constant at about 40ml. |
|
interpretation 1: |
Fe + O2
---> FeO
iron oxygen iron(II)-oxide |
|
procedure 2: |
Fill the remaining gas into the test tube and put a burning wooden
splint into the gas.
|
|
observation 2: |
The flame goes
out. |
|
interpretation 2: |
There is no (not
enough) oxygen in the gas for combustion. Air contains about 20 percent oxygen. |
|
source: |
Barke, H.-D. et al.: One Hundred
Chemistry-Experiments to Avoid Chalk and Talk; University of Muenster,
2004. |