| time
requ.: |
5 minutes. |
|
objective: |
Connection between
oxidation and
air. |
| material: |
|
|
| chemicals: |
|
- copper foil 10*10cm,
thickness 0,15mm
|
|
procedure 1: |
Heat the little piece of copper
foil in the uppermost part of the burner flame (oxidizing part). |
|
observation 1: |
The foil
becomes
black. |
|
hypothesis 1: |
For oxidation
you need a flame. |
|
procedure 2: |
Make a little "letter" out of
the square shaped copper foil:
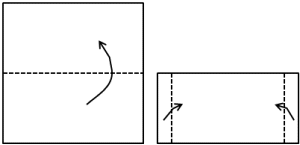
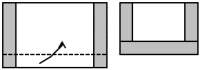
Heat the "letter" in the
uppermost part (oxidizing) of the flame for a few seconds (til
colour gets darker) and open it again (after cooling down!). |
|
observation 2: |
The outer sides change
to brown or black,
the inner sides mostly show the original red colour. |
|
hypothesis 2: |
For oxidation
a flame (heat) and oxygen are
necessary. |
|
interpretation: |
2 Cu + O2
---> 2 CuO
copper(0) oxygen copper(II)-oxide
Copper reacts with oxygen to form copper oxide. |
|
source: |
Barke, H.-D. et al.: One Hundred
Chemistry-Experiments to Avoid Chalk and Talk; University of Muenster,
2004. |
|
comment: |
Hypothesis 1
was not sufficient to explain observation 2. The model for oxidation had
to be expanded. |